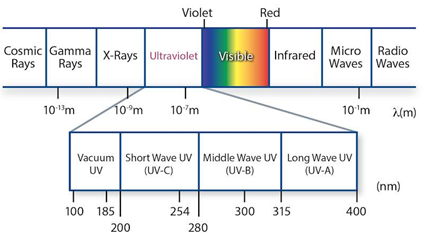
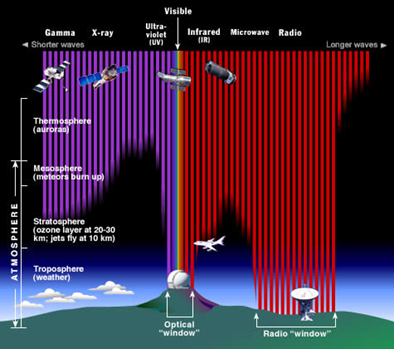
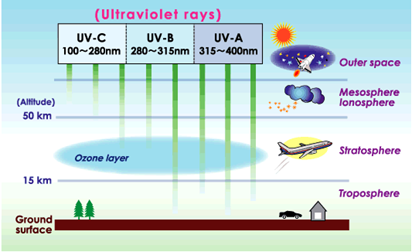
BLOGGING FOR PLANET EARTH
The written and creative works in this website are licensed under a
Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License
Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License